The National Medical Products Administration approved the IND of Sino-US Group's NK immune cell new drug, opening a new era of tumor treatment
TIME:2025-06-23 Views:110
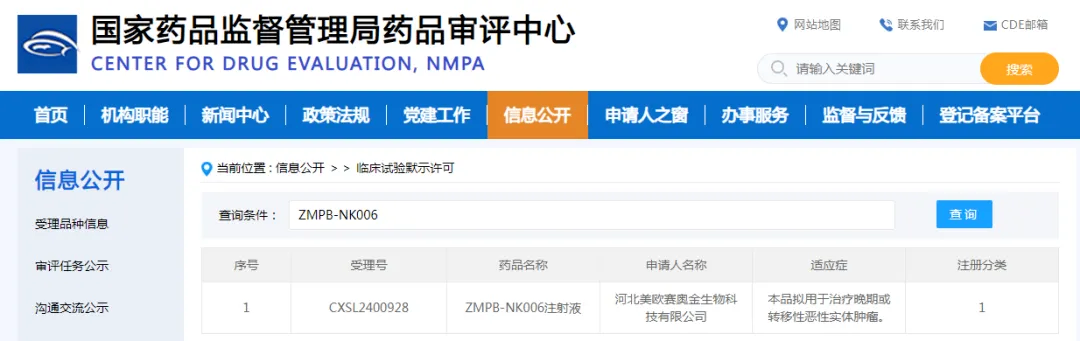
Recently, the official website of the Center for Drug Evaluation (CDE) of the National Medical Products Administration released an exciting news: the ZMPB-NK006 injection applied by Hebei Meiosai Aojin Biotechnology Co., Ltd. has officially obtained the implied approval for clinical trials (IND). This marks that the first spot NK cell therapy in China is about to enter the clinical trial stage, bringing unprecedented new hope for the treatment of advanced or metastatic malignant solid tumors.
Natural killer cells (NK cells), as an important member of the body's immune system, play a vital role in the fight against cancer. They can accurately identify specific molecular markers on the surface of tumor cells, such as tumor-associated antigens, and directly bind to tumor cells. Subsequently, NK cells release cytotoxic substances such as perforins and granzymes, which penetrate the cell membrane of tumor cells and induce their apoptosis. This process not only directly eliminates tumor cells, but also demonstrates the powerful power of NK cells in the fight against cancer.
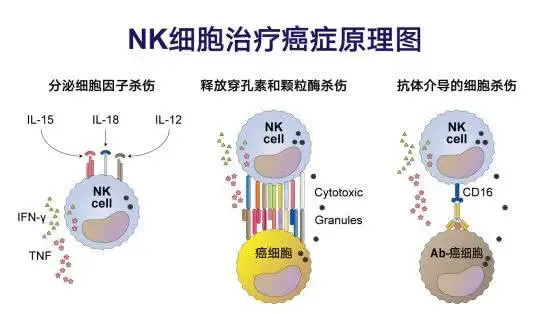
In addition to directly killing tumor cells, NK cells also have the ability to secrete a variety of cytokines, such as interferon-γ, tumor necrosis factor-α, etc. These cytokines can not only directly inhibit the growth and proliferation of tumor cells, but also regulate the body's immune microenvironment and enhance the killing effect of other immune cells (such as T cells and macrophages) on tumor cells. This synergistic anti-cancer effect makes NK cells show great potential and broad application prospects in tumor treatment.
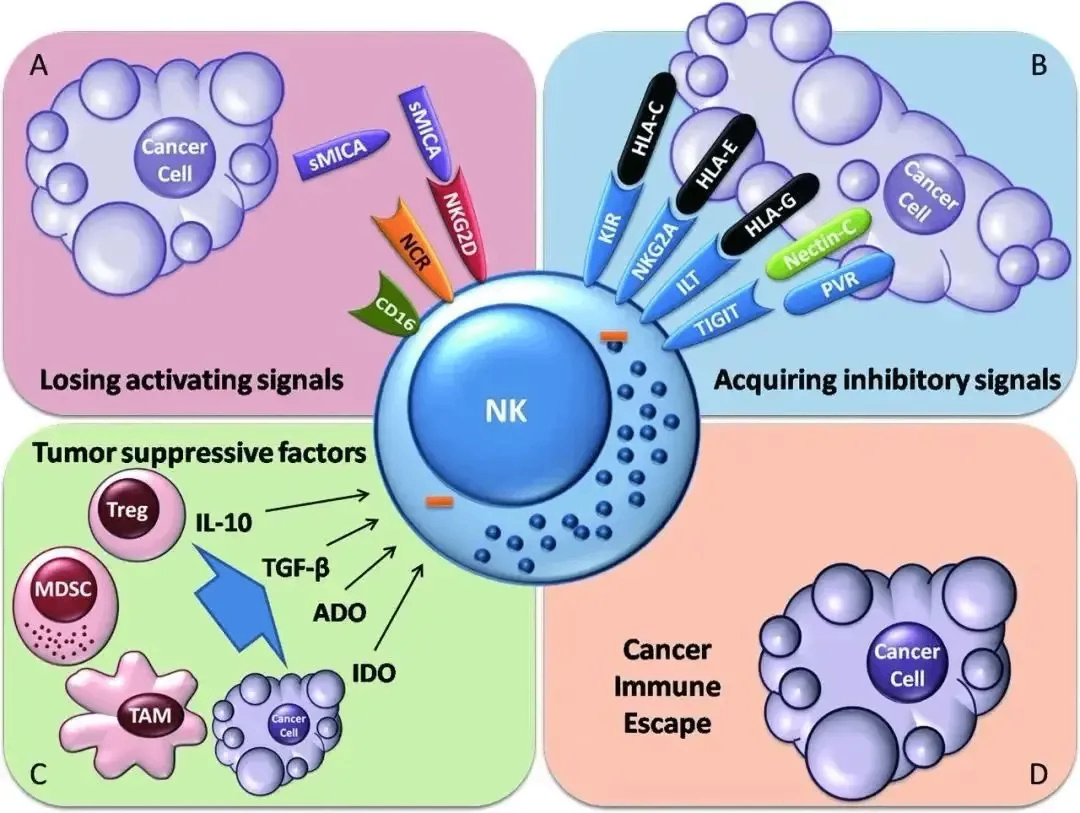
As a leader in cell biotransformation applications, the American Ceokin Group and its Chinese partner, Sino-US Ceokin, have been deeply engaged in cell storage, cell biotransformation and the bio-health industry chain since their establishment, and have continuously made breakthrough progress.
-
Prev:No More Post